Clinical Outcomes of Next-Generation Chimeric Antigen Receptor T-Cell Therapy in Acute Lymphoblastic Leukemia
Authors
##plugins.themes.bootstrap3.article.main##
Abstract
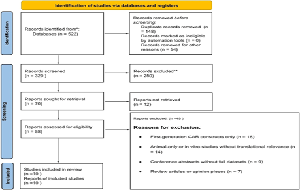
Objective: To evaluate efficacy, durability, and safety of next-generation chimeric antigen receptor T-cell therapies in relapsed or refractory acute lymphoblastic leukemia. Design: A systematic review following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines with a predefined protocol. Subjects/Patients: Pediatric and adult patients with relapsed or refractory acute lymphoblastic leukemia treated with next-generation chimeric antigen receptor T-cell therapies using dual or multi-antigen targeting, cytokine-armored constructs, or logic-gated systems. Ten open-access studies including 327 heavily pretreated patients were analyzed. Methods: Electronic databases were systematically searched for eligible studies. Extracted data included patient characteristics, chimeric antigen receptor design, remission rates, durability, relapse patterns, and toxicity. Risk of bias was assessed. Results: Complete remission rates ranged from seventy-eight percent to ninety-two percent, exceeding historical outcomes. Dual or multi-antigen targeting reduced antigen-negative relapse to less than ten percent. Cytokine-armored constructs showed enhanced persistence and prolonged remission, with engineered T cells detectable beyond six months in over half of patients. Logic-gated systems showed comparable efficacy with improved specificity. Cytokine release syndrome and neurotoxicity were manageable. Conclusion: Next-generation chimeric antigen receptor T-cell therapies demonstrate robust efficacy, improved durability, and acceptable safety, representing a meaningful evolution of adoptive cellular immunotherapy requiring validation in larger standardized trials.
##plugins.themes.bootstrap3.article.details##
Copyright (c) 2026 Dr. Birupaksha Biswas, MD, Dr. Suhena Sarkar, MD

This work is licensed under a Creative Commons Attribution 4.0 International License.
Creative Commons License All articles published in Annals of Medicine and Medical Sciences are licensed under a Creative Commons Attribution 4.0 International License.
[1] Shannon L. Maude, Theodore W. Laetsch, Jochen Buechner, et al.: Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 439-448. 2018, VOL. 378 NO. 5:439-448. 10.1056/NEJMoa1709866
[2] Jae H. Park, Isabelle Rivière, Mithat Gonen, et al.: Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018, VOL. 378 NO. 5:449-459. 10.1056/NEJMoa1709919
[3] Shah, N.N., Fry, T.J: Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 16:372-385.
[4] Pan J, Niu Q, Deng B, et al.: CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019, 33:2854-2866. 10.1038/s41375-019-0488-7
[5] Adachi K, Kano Y, Nagai T, et al.: IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor.. Nature biotechnology. 2018, 36:346-351. 10.1038/nbt.4086
[6] Hurton LV, Singh, H Najjar, et al.: Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proceedings of the National Academy of Sciences of the United States of America. 2016, 113(48):E7788-E7797. 10.1073/pnas.1610544113
[7] Roybal KT, Rupp LJ, Morsut L, et al.: Precision Tumor Recognition by T Cells with Combinatorial Antigen- Sensing Circuits. Cell. 2016, 164(4):770-779. 10.1016/j.cell.2016.01.011
[8] Locke F L, Ghobadi A, Jacobson, et al.: Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. The Lancet. Oncology. 2019, 20(1):31-42. 10.1016/S1470-2045(18)30864-7
[9] Neelapu S, Tummala S, Kebriaei, et al.: Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit 'ALL'. Nat Rev Clin Oncol. 2018, 15:218. 10.1038/nrclinonc.2018.20
[10] Zah E, Lin M Y, Silva-Benedict, et al.: T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer immunology research. 2016, 4(6):498-508. 10.1158/2326-6066
[11] Shirzadian M, Moori S, Rabbani R, et al.: SynNotch CAR-T cell, when synthetic biology and immunology meet again. Front Immunol. 2025, 16: 10.3389/fimmu.2025.1545270
[12] Majzner, R.G., Mackall, C.L: Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med. 25:1341-1355.
[13] Orlando EJ, Han X, Tribouley C, et al.: Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018, 24(10):1504-1506. 10.1038/s41591-018-0146-z
[14] Fischer J, Paret C, El Malki K, et al.: Isoforms Enabling Resistance to CART-19 Immunotherapy Are Expressed in B-ALL Patients at Initial Diagnosis. J Immunother. 2017, 40(5):187-195. 10.1097/CJI.0000000000000169
[15] Ruella M, Maus MV, M.R, et al.: Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies. Comput Struct Biotechnol J. 2016, 14:357-362. 10.1016/j.csbj.2016.09.003
[16] Grada Z, Hegde M, Byrd T, et al.: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids. 2013, 2(7):e105. 10.1038/mtna.2013.32
[17] Wherry EJ, Kurachi M, J. Kurachi, et al.: Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015, 15(8):486-99. 10.1038/nri3862
[18] Lynn RC, Weber EW, Sotillo E, et al.: c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019, 576(7786):293-300. 10.1038/s41586-019-1805-z
[19] Kagoya Y, Tanaka S, Guo T, et al.: A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018, 24(3):352-359. 10.1038/nm.4478
[20] Xu Y, Zhang M, Ramos CA, et al.: Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014, 23(24):3750-9. 10.1182/blood-2014- 01-552174
[21] Morsut L, Roybal KT, Xiong X, et al.: Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell. 2016, 164(4):780-91. 10.1016/j.cell.2016.01.012
[22] Roybal KT, Lim WA, W.A.L, et al.: Synthetic Immunology: Hacking Immune Cells to Expand Their Therapeutic Capabilities. Annu Rev Immunol. 2017, 35:229-253. 10.1146/annurev-immunol-051116-052302
[23] Srivastava S, Riddell SR, Engineering CAR-T cells: Design concepts. Trends Immunol. 2015, 36(8):494-502. 10.1016/j.it.2015.06.004
[24] Hay KA, Hanafi LA, Li D, et al.: Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017, 130(21):2295-2306. 10.1182/blood-2017- 06-793141
[25] Gust J, Hay KA, Hanafi LA, et al.: Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7(12):1404-1419.10.1158/2159-8290.CD-17-0698
[26] Depil S, Duchateau P, Grupp SA, et al.: 'Off-the-shelf' allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020, 19(3):185-199. 10.1038/s41573-019-0051-2
[27] June CH, Sadelain M, Carl H. June, et al.: Chimeric Antigen Receptor Therapy. N Engl J Med. 2018, 379(1):64-73. 10.1056/NEJMra1706169.